







Streptococcus equi subsp. equi (henceforth termed S. equi) is responsible for the clinical condition known colloquially as ‘strangles’, a highly contagious disease that is endemic worldwide with only a handful of countries being free of the condition. Fatality rates have been reported at between 1 and 10%, and rates of morbidity are far higher (Boyle et al, 2018). Strangles causes profound disruption and economic losses to the equine industry, and is one of the most challenging equine infectious diseases to manage.
Epidemiology
S. equi is an obligate pathogen that does not survive well outside the horse. Elimination of the disease should be a realistic aim; in some countries, strangles is a reportable or notifiable disease. A major factor in S. equi's success as an equine pathogen is its ability to survive in, and spread from, horses that are not exhibiting clinical signs. Transmission from outwardly healthy horses is likely to be of greater importance than transmission from horses with clinical signs; therefore, identification of these horses and elimination of infection is critical in preventing new outbreaks.
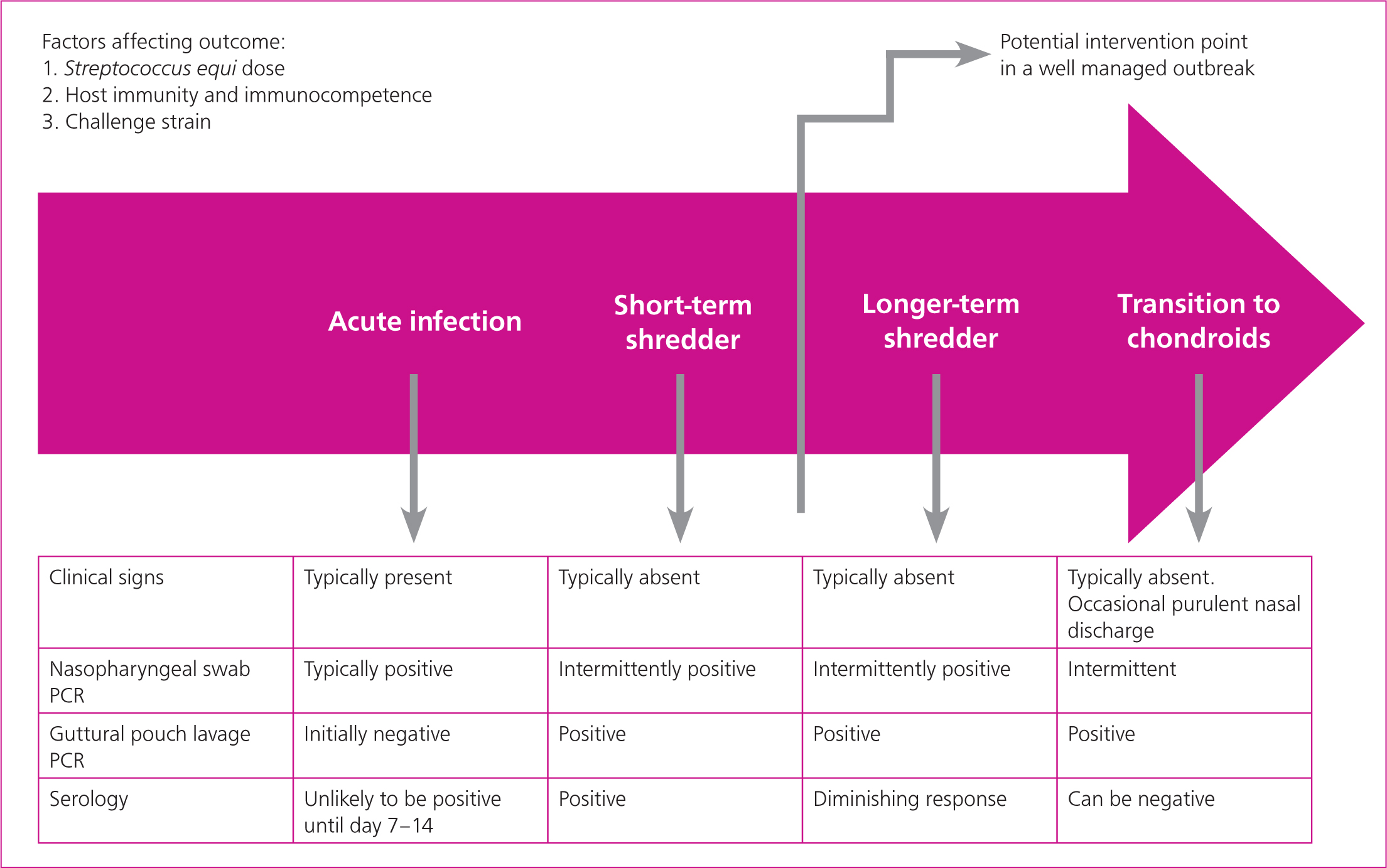
Following exposure to S. equi there is an incubation period typically lasting up to 14 days. The duration of the incubation period will be influenced by pre-existing immunity in the horse and the size of the dose of S. equi received. It may be as short as a day with high dose experimental challenge or possibly as long as 28 days with lower natural challenge doses in the field and some immunity from previous infection. Following exposure, rectal temperature typically increases before the horse becomes infectious and this can be utilised in the control of the disease by segregating cases before they become infectious (see ‘outbreak management’ below).
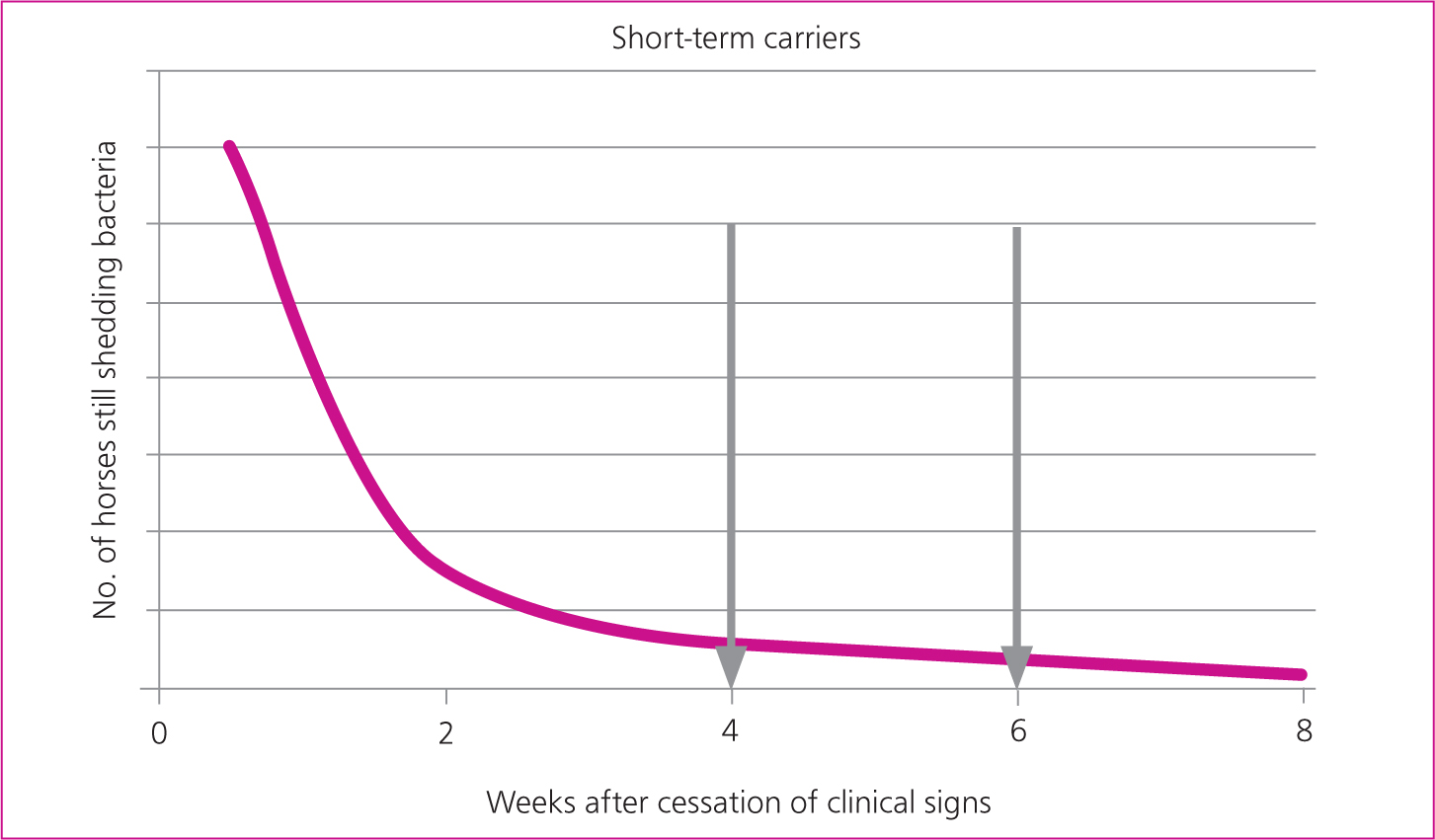
Horses that have shown clinical signs of disease may remain infectious for weeks after the cessation of clinical signs and this presents a risk for onward transmission if freedom from infection is not demonstrated prior to lifting isolation measures on each case. A previous expert consensus recommended that horses are considered infectious for 6 weeks after the cessation of nasal discharge (Boyle et al, 2018). In practice, the duration of infection and contagion may be longer than this.
Approximately 75% of outbreaks result in the development of ‘carrier’ horses in which infection may persist within one or both guttural pouches (Newton et al, 1999) or, rarely, in the nasal sinuses. S. equi is not unique in causing lymph node abscessation and subsequent guttural pouch empyema. While it is uncommon, guttural pouch empyema may also result from infection with Streptococcus zooepidemicus, a closely related bacterium with which S. equi shares 97% genetic homology (Laus et al, 2007).
The severity of clinical signs will be influenced by immune status and immunocompetence. Younger horses, particularly foals and weanlings, are more vulnerable to infection with S. equi, and morbidity and mortality will be higher than in adult horses. Recent surveillance based on laboratory detection of S. equi in 2019 and 2020 has indicated that the median age of horses infected with strangles in the UK is 9 years (McGlennon, unpublished data, www.jdata.co.za/ses ). This is older than might be expected, considering the traditional perception that strangles is a disease that primarily affects younger horses. However, these data include older subclinical carriers in which S. equi infection has also been confirmed. A greater number of reports come from commercial compared to private yards and more outbreaks are reported in the summer compared to the winter (McGlennon, unpublished data). Weaning and mixing of young horses are believed to be particularly high-risk events for the spread of strangles.
Movement of fomites within, and potentially between, yards presents a potential route of disease transmission, although S. equi does not survive for long in the presence of competing environmental bacteria. Investigations that have attempted to simulate environmental contamination have indicated that S. equi may survive for up to 3 days on fencing or soil but for less than 24 hours on wood, metal and rubber surfaces in direct sunlight (Weese et al, 2009). By contrast, another study indicated that colonies inoculated into the environment could survive for up to 34 days, with survival times being longer in a wet environment and winter season (Durham et al, 2018). Water troughs or drinkers present a particular risk as S. equi will survive in water for up to 4–6 weeks (Boyle et al, 2018). While elimination of S. equi from the environment is an important consideration, it is unknown how important environmental persistence is in the epidemiology of strangles.
Pathogenesis
S. equi attaches to, and then penetrates, the lymphoid tissues of the upper airway. Within a few hours of exposure, S. equi will often not be detectable on the mucosal surface (rendering diagnostic testing futile) and will have translocated to lymph nodes where complement interactions with bacterial peptidoglycan and the production of superantigens trigger the release of chemotactic factors that attract neutrophils. S. equi's hyaluronic acid capsule and surface proteins (including SeM) impede phagocytosis by neutrophils which accumulate, resulting in enlarging abscesses that, ultimately, rupture releasing bacteria and facilitating onward transmission. Nasal shedding typically develops 2–3 days after the onset of pyrexia and generally lasts 2–3 weeks; however, shedding can occur for months and potentially years if infection persists in the guttural pouches. In horses that clear infection, cessation of discharge and shedding coincides with the development of systemic and mucosal immune responses.
Experimental challenge studies suggest that at least 10 000 colony forming units are required to cause disease in immunocompetent adult horses. Any less, and infection is likely to be eliminated by immune defenses either at the mucosa or in lymph nodes (Boyle et al, 2018). As the challenge dose increases, so the incubation period shortens and the likely severity of clinical signs increases. Unfortunately, the timing and magnitude of challenge dose are indeterminable in naturally occurring infections.
If the course of disease progresses without the administration of antimicrobials, 75% of horses are expected to develop immunity to re-infection (Hamlen et al, 1994), which leaves 25% at risk of re-infection. Immunity wanes over time in the absence of re-exposure. Paradoxically, in some populations, chronic carriers may help to maintain immunity and reduce recurrence of clinical disease, although the infection will then remain endemic.
Maternally derived immunity provides a degree of protection if mares have a history of recent exposure. Colostral antibodies from exposed mares are found to re-circulate to the mucosa of the nasopharynx providing some protection for the first few weeks of life; thereafter, IgGb and IgA antibodies ingested in milk will provide some protection through to weaning (Galan et al, 1986).
Clinical signs
Most reported cases of strangles have non-specific signs of upper respiratory tract infection and/or pyrexia (McGlennon, unpublished data). A minority of cases exhibit the ‘typical’ and emotive signs of lymph node abscessation and dyspnoea. The misleadingly termed ‘atypical strangles’ cases that have non-specific signs of nasal discharge, pyrexia and mild lymphadenopathy should not be overlooked and it is recommended that S. equi is included whenc panels of laboratory tests are used to screen for infectious respiratory disease either by enzyme-linked immunosorbent assay (iELISA) or polymerase chain reaction (PCR). The apparent increase in frequency of ‘atypical strangles’ cases makes them much less ‘atypical’ and might be due to differences in circulating strains of S. equi. However, improvement in diagnostic tests may now allow identification of cases that would previously have gone undiagnosed.
With ‘typical’ cases, pyrexia and lethargy manifest 3–14 days after infection as pharyngitis and lymph node abscessation develop. Abscessation occurs in decreasing order of frequency in the retropharyngeal, submandibular, parotid and cranial cervical lymph nodes. Lymph node abscesses may rupture, (generally 1–4 weeks after infection) into the guttural pouch or through the skin, depending on which lymph nodes are affected. Abscess rupture often coincides with resolution of pyrexia and improved demeanour. Cough and nasal discharge may develop in association with pharyngitis. Nasal discharge may be marked in association with abscess rupture and guttural pouch empyema.
Discharge is typically bilateral but may be unilateral if only one guttural pouch is affected. Ocular swelling, as a result of parotid or retrobulbar abscessation, and conjunctivitis are less common clinical signs. In rare cases, sinusitis may occur. Lymphadenopathy and respiratory tract inflammation may result in respiratory tract obstruction and signs of dyspnoea or dysphagia. Neuropraxia or permanent nerve damage, usually secondary to guttural pouch empyema, can result in laryngeal or pharyngeal dysfunction contributing to dysphagia. Purpura haemorrhagica, myositis and myocarditis are uncommon sequelae. The frequency of different clinical signs reported to the Surveillance of Equine Strangles network is shown in Figure 1.

Rarely, lymph nodes not associated with the upper respiratory tract may be affected, giving rise to the questionably termed ‘bastard strangles’, although the authors suggest that ‘disseminated streptococcal lymphadenopathy’ would be a more appropriate description. Infection of the abdominal and thoracic lymph nodes, lungs, peritoneal and thoracic cavities, brain, and mammary gland have been reported (Boyle et al, 2018).
Diagnosis
Sampling
Appropriate timing of diagnostic testing is essential (Figures 2a and 2b). Following exposure, S. equi is unlikely to be detectable, either by culture or PCR of swabs or guttural pouch washes, until lymph nodes rupture, which typically takes 1 to 4 weeks. Once there is discharge, swabbing can be performed to collect samples for culture and PCR. Nasopharyngeal swabs that sample from more of the upper respiratory tract are recommended for diagnosis of the acute case and can be obtained from: equinesurveillance@gmail.com. Short cottontip swabs passed just inside the nares are less reliable and are more likely to be contaminated with other (environmental) bacteria; they should only be used if there is a copious purulent nasal discharge. Nasal lavage may also be performed and in one study was shown to have greater sensitivity than swabs (Lindahl et al, 2013); however, the swabs used had a far smaller surface area than those used in the UK.
With nasal lavage there would seem to be a greater risk of contamination of the environment and attending personnel, at least in less experienced hands. If lymphadenopathy is present, a diagnosis may be possible before lymph node rupture with PCR of an aspirate from an enlarged lymph node. A negative result on a lymph node aspirate does not provide confirmation that there is freedom from infection. If negative results conflict with the presence of suggestive clinical signs or a high level of suspicion of exposure to S. equi infection then repeated sampling is prudent. ‘If it looks like strangles then it probably is strangles’ is a useful adage to follow. Endoscopy can often provide useful information on the presence and stage of infection.
Culture and sugar fermentation
Traditional bacterial culture methods can be used to confirm the presence of streptococci and sugar fermentation tests applied to confirm the species present. However, traditional culture methods have a number of disadvantages: i) bacterial culture is far less sensitive than PCR; ii) false negative results may occur if organisms present in samples are not viable when they reach the laboratory; iii) S. zooepidemicus is a common opportunistic pathogen and if mixed β-haemolytic streptococcal infections are present, S. equi may be overlooked; and iv) it takes a number of days to obtain results. On rare occasions, culture may identify the presence of S. equi when PCR has failed and false negative PCR results have been obtained; however, this scenario is so infrequent that culture is increasingly omitted when concurrent PCR is being performed.
Polymerase chain reaction
PCR usually provides results within 24 hours (and potentially far less) which represents a significant advantage over traditional culture results in preventing disease spread. Early PCR methods were targeted at the gene encoding the SeM protein. However, this gene exhibits variation, with segments being truncated in carrier horses (Chanter et al, 2000; Anzai et al, 2005). Other, more consistent targets have since been identified. Webb et al (2013) developed an assay that had two gene targets (eqbE, SEQ2190) and a synthetic DNA sequence (SZIC) acting as an internal control. Other PCR methods have also been developed with a single target gene (North et al, 2014; Cordoni et al, 2015); with a single target there is a greater possibility of false negative results in the event of gene deletion.
The different gene targets used by commercial laboratories in the UK are shown in Table 1. As PCR methods become less expensive to develop and use, alternatives are being brought to the market which will be advantageous to disease control, provided that they are appropriately validated and are accurate. Loop-mediated isothermal amplification (LAMP) assays that allow DNA amplification and detection in a single step have been developed for rapidly detecting the SeM (Hobo et al, 2012) and eqbE targets (North et al, 2014). These assays are appealing, although in a comparative study the eqbE LAMP assay had 11% lower sensitivity and 21% lower specificity when compared against the eqbE PCR assay (Boyle et al, 2017), which undermines confidence in the methodology compared to laboratory performed PCR. LAMP assay technology continues to improve and further advances in this field are expected in the coming years.
Table 1. PCR and ELISA tests for Streptococcus equi used by five* of the major commercial laboratories in the UK
| PCR gene target | ELISA | |||||||
|---|---|---|---|---|---|---|---|---|
| seel | sodA | eqbE | SEQ2190 | Internal PCR control | Duplex | SeM | ||
| LABORATORY* | A | ✓ | ✓ | ✓(alternate§) | ✓(alternate§) | ✓ | ✓ | |
| B | ✓ | ✓ | ✓ | ✓ | ||||
| C | ✓ | ✓ | ✓ | ✓ | ||||
| D | Not disclosed | ✓ | ||||||
| E | ✓ | ✓ | ✓ | ✓ | ||||
PCR = polymerase chain reaction, ELISA = enzyme-linked immunosorbent assay.
* Five laboratories, that between them perform the bulk of ‘strangles’ testing in the UK, were approached by the authors and four provided details of the tests they perform §Not typically used first-line but available when indicated
Anyone submitting samples for S. equi PCR should be aware of the diagnostic accuracy of the test that is being used and that not all tests are ‘created equal’. The accuracy of PCR methods is dependent on scrupulous technique and the elimination of any possibility of cross-contamination in the laboratory or other environment where they are operating.
Interpretation of PCR results is also important as the results generated are not dichotomous and careful consideration has to be given to results with low DNA copy or high cycle threshold (CT) numbers that suggest the presence of low levels of target organism that might not be relevant clinically or, indeed, accurate if attributable to cross-contamination. The inclusion of a positive control DNA target provides greater reassurance that negative results are genuine and are not the result of a failure of the laboratory process. Higher laboratory throughput and processing of greater numbers of positive samples increases the risk of false positive results due to contamination, so quality assurance becomes increasingly important. A quality assurance scheme is available for strangles diagnosis by S. equi detection and clinicians should be wary of using laboratories that are not Vetqas accredited for such methods (http://apha.defra.gov.uk/ahvla-scientific/vetqas/PT0193.html ).
False positive PCR diagnoses as a result of carryover of non-viable organisms or degrading DNA is a risk when groups of horses are being examined by endoscopy. High CT values, equating to very small numbers of bacteria, have to be interpreted carefully within the laboratory. The authors caution about using low level PCR results as the sole basis for defining S. equi carriers. Veterinary surgeons should be aware of the potential for this ‘carryover’ phenomenon and the following precautions are advised:
- Make a note of the order in which animals and sides of the head in each animal are sampled as this will allow any pattern of possible carryover to be assessed and linked to extent of pathology encountered during sampling.
- Schedule sampling of horses in reverse order of their perceived risk of having active S. equi infection; i.e. look to sample those horses with highest likelihood of being positive later/last.
- Consider cessation of sampling for the session pending thorough cleaning and disinfection if an overtly diseased animal is encountered and there is believed to be a high risk of cross-contamination.
- Request that laboratories indicate quantitatively the bacterial load found in any positive samples (ideally as a copy number or alternatively as a CT value); this will, again, allow any pattern of positivity to be better assessed.
- Always repeat sample animals that give rise to unexpected low level positive PCR results rather than assuming they are false positives that are not infectious.
iELISA
Measurement of the antibody response to S. equi enables horses that have been exposed to S. equi to be identified. Serological testing was first developed to identify horses with high levels of SeM-specific antibodies to detect potential complications, such as metastatic abscesses or the risk of developing purpura haemorrhagica if vaccinated with SeM-containing vaccines (Sweeney et al, 2005). However, cross-reactivity between the SeM protein and the homologues SzM in S. zooepidemicus (Kelly et al, 2006) resulted in false positive diagnoses of strangles. Therefore, a dual antigen iELISA was developed targeting antibodies to the N-terminal portion of SEQ2190 surface protein (antigen A) in addition to the N-terminal portion of the SeM protein which is unique to S. equi (antigen C) (Robinson et al, 2013). Both the dual antigen iELISA and an SeM iELISA are available commercially in the UK (Table 1) and were compared during the development of the dual antigen iELISA. The SeM iELISA had a sensitivity of 89.9% and specificity of 77%, whereas the dual antigen iELISA had a sensitivity of 93.9% and a specificity of 99.3%. Therefore, the single SeM iELISA incorrectly identified 23% of sera from horses known to have never been exposed to S. equi as positive for S. equi antigens (Robinson et al, 2013). The dual antigen and SeM iELISA tests have been compared in France where the strains circulating are similar to the UK. The dual antigen iELISA detected 70% of horses that had been infected >70 days prior, while the SeM iELISA only detected 40% (Albertine Leon, LABÉO, France, unpublished data). A quality assurance scheme is available for strangles serology and clinicians should be wary of using laboratories that are not Vetqas accredited for such methods (http://apha.defra.gov.uk/ahvla-scientific/vetqas/PT0175.html ).
Serological testing is best used in the immediate aftermath of an outbreak to differentiate horses that have been exposed and might be persistently infected from those that have not been exposed and do not present a risk of being carriers. Potential carriers can be investigated further with guttural pouch lavage and submission of the resultant samples for PCR testing.
The use of serology as a screening test for chronic carriers has become commonplace (Waller, 2014). However, with chronicity of infection, the serological test appears to become less reliable as not all horses maintain high concentrations of antibodies to S. equi, even if there is persistent infection within the guttural pouches. This is thought to be due to genetic decay and a reversion away from virulence in some of the S. equi strains maintained in guttural pouch empyema (Harris et al, 2015; Boyle et al, 2017). Recent publications have highlighted failures in the ability of the dual antigen iELISA test to identify all horses that have guttural pouch empyema or S. equi DNA within the guttural pouch (Bowen et al, 2020; Durham and Kemp-Symonds, 2021). Another recent investigation demonstrated that 75% of ‘carriers’ were identified for up to 10 months using the dual antigen iELISA test (Pringle et al, 2020) and the value of the test for identifying carriers has been demonstrated in practice (Knowles et al, 2010). This rate of carrier detection is supported by unpublished data from France in which the dual antigen iELISA detected 70% of horses that had been infected >70 days prior (Albertine Leon, LABÉO, France, unpublished data). The rate of 70% was lower than at early time points; within 7 days of the onset of clinical signs the rate of detection was 81% with the rate of detection being highest 15–21 days after the onset of clinical signs at 86%. This is consistent with serology being most valuable when performed in the immediate aftermath of an outbreak.
While comparing results of ELISA and PCR tests over-simplifies the challenges of detecting carriers that present a risk for transmission of infection, these recent investigations of the dual antigen iELISA in detecting chronic carriers have highlighted the misguided inference of some laboratories and clinicians in definitively confirming the absence of a carrier state based solely on a single or paired negative iELISA result. Identification of carriers is not ‘black and white’, and factors such as how carriers are being defined, clinical and outbreak history, use of antimicrobials, individual response to infection and immunity have to be considered, alongside recognising the potential for genetic variability of carrier strains.
Subject to these limitations, the authors believe the iELISA remains a useful tool on both a population and single horse basis albeit that it may miss long-term carriers (Pringle et al, 2020). It would be preferable for all potential carriers to be examined by guttural pouch endoscopy and sampling, but this is unlikely to happen in practice and the use of serology remains better than the absence of investigation of carrier status as long as limitations are understood by veterinary surgeons and yard owners.
Of laboratory confirmed S. equi positive cases submitted to the Surveillance of Equine Strangles network between 2019 and 2020, 10% of infected horses were diagnosed following initial positive serology results (McGlennon, unpublished data). Use of a mandatory testing protocol prior to entry to a yard or country also serves as a deterrent to those who might otherwise consider moving a horse that is known to have been exposed to strangles and has not been confirmed to be free of infection.
Discontinuing the use of serological testing could significantly set back efforts to prevent the spread of strangles. Where the iELISA test fails to identify horses that have guttural pouch empyema, guttural pouch samples or chondroids themselves should be submitted for PCR and culture. To facilitate further investigation of this phenomenon in order to try to improve the performance of serological testing, surplus sera and S. equi cultures should be retained (frozen at -20°C and, ideally, including multiple selected colonies) from these seronegative, guttural pouch culture-positive animals.
It is important to appreciate that the SeM iELISA is subject to the same limitations in detecting chronic carriers as the dual antigen iELISA and suffers from the additional limitations of i) cross-reactivity with S. zooepidemicus and ii) the failure to detect strains in which genes encoding the relevant protein have been deleted (Kelly et al, 2006; Paillot et al, 2010; Harris et al, 2015). The strains circulating in Europe have changed over recent years, coinciding with decreased immune responses to SeM, and suggesting that their expression of this important protein may be reduced (Mitchell et al, 2021). A review of recent culture- or PCR-positive samples that had been submitted to the AHT, where the sampled horse also had a serology result within the previous or following 2 weeks indicated that the antigen C assay (SeM) identified only 17% of these samples (Waller, unpublished data). A reduced reactivity to SeM was also apparent in France where 77.5% of serum samples from 18 outbreaks of strangles tested positive in the dual antigen iELISA, of which 98% and 39% of these seropositive samples were identified by the antigen A or C assays, respectively. The greater reliance on the ‘A’ antigen in the dual antigen iELISA has identified a need to replace the SeM-component of this assay with an alternative antigen that has a higher sensitivity for the detection of horses that have been exposed to contemporary strains of S. equi. In the same study, only 56.7% of the French serum samples from 18 strangles outbreaks tested positive using the IDVet SeM iELISA (compared to 77.5% using the dual antigen iELISA), highlighting the reduced sensitivity of SeM-based assays (Albertine Leon, LABÉO, France, unpublished data).
When performing diagnostics for S. equi in horses with signs of strangles, the possibility of infection with highly pathogenic strains of S. zooepidemicus expressing superantigens always needs to be considered. In this situation, with horses showing signs of strangles, the differentiation of S. equi and S. zooepidemicus is academic as virulent strains of S. zooepidemicus with a propensity to produce lymph node abscessation should be managed in the same way as S. equi. Over 400 strains of S. zooepidemicus have been identified, with around a third producing superantigens; however, only a minority will cause lymph node abscessation and empyema (Paillot et al, 2010; Rash et al, 2014).
Case management
Acute infection
The severity of clinical signs that develop following infection with S. equi will be dependent on several factors including i) challenge dose, ii) host immunity and immunocompetence, and iii) challenge strain. Most cases that are infected with S. equi exhibit mild, non-specific signs of acute respiratory infection and respond well with minimal supportive and nursing care. A proportion of cases require more supportive care, and a few require intensive treatment that may be better offered in a hospital setting.
Non-steroidal anti-inflammatory drugs (NSAIDs) may be administered to provide analgesia, combat pyrexia and improve appetite and welfare. It has been suggested that NSAIDs might slow the development of abscesses through their anti-inflammatory effects; however, abscesses are likely to develop and rupture, irrespective of such treatment. The use of acetaminophen (paracetamol) might be considered as it has analgesic and anti-pyretic effects without inhibiting inflammation. There is, however, no evidence of its beneficial use in strangles. Soft, palatable, nutrient-dense feed will help to encourage eating, and providing feed and water from a height may help horses with marked lymphadenopathy. The application of ‘hot-packs’ to abscesses may help them to mature and rupture more quickly or abscesses may be opened surgically. Once abscesses are open, they can be lavaged with saline or a dilute antiseptic solution. In rare cases, fluid therapy and even supplementary enteral or parenteral nutrition may be required for horses that have severe lymphadenopathy; the prognosis for these cases is guarded. Acute cases of strangles can develop and change rapidly and regular monitoring and nursing care are essential.
The use of antimicrobials in acute strangles cases is controversial. Antimicrobial use has been advocated following exposure and prior to abscessation to prevent the development of abscesses (Boyle et al, 2018). With a high challenge dose, abscesses can develop rapidly within a couple of days, so unless antimicrobials are used immediately after exposure there is a risk that the use of antimicrobials may simply delay the development of abscesses. The use of antimicrobials has been shown to suppress serological responses (Pringle et al, 2019), indicating that they may also compromise subsequent immunity to reinfection.
In the absence of evidence that antimicrobials are of benefit in preventing lymphadenopathy they should be avoided in this scenario. In horses with dyspnoea, dysphagia or marked persistent pyrexia the use of antimicrobials is indicated on welfare grounds. Different abscesses may mature at different rates, so while the use of antimicrobials might be considered to speed the resolution of draining abscesses, they might also be inhibiting the development of others and ultimately protracting the course of the disease. If lymphadenopathy is severe or there is laryngeal paralysis, tracheostomy may be required. Prognosis in these cases is poor as laryngeal paralysis is often permanent.
Hygiene is essential when managing horses with strangles and every effort should be made to reduce the risk of environmental contamination. This can be challenging when large volume lavage is being performed, but every attempt should be made to catch and dispose of infected material. Having horses well sedated reduces the risk of harm to both the horse and attending personnel and makes it easier to collect infectious material.
Investigation and treatment of guttural pouch empyema
It is estimated that without intervention, an average of 10% of horses exposed to S. equi would go on to be persistently infected and would serve as a source of infection for other horses (Newton et al, 2000). This figure will vary markedly between outbreaks. Guttural pouch empyema has a variable time course but most chronic cases will require intervention for infection to resolve. The timing of investigation for persistently infected horses is a balance between the desire to resolve the outbreak quickly and the costs associated with identifying, treating and retesting greater numbers of PCR positive horses (Figure 2b). The sooner investigation is performed, the more likely it is horses will be identified as infected and will require further investigation and treatment, increasing the costs of veterinary intervention. In one study the median duration of positivity on guttural pouch samples was 60 days (interquartile range 40–75 days) (Duffee et al, 2015).
Demonstration that guttural pouch lavage samples are negative on PCR is the only reliable means of demonstrating freedom from infection. It is possible that DNA will be present even when no viable S. equi remain; however, the authors consider that DNA is degraded and cleared from the guttural pouch within days of cessation of infection so horses that are PCR positive should be considered infectious.
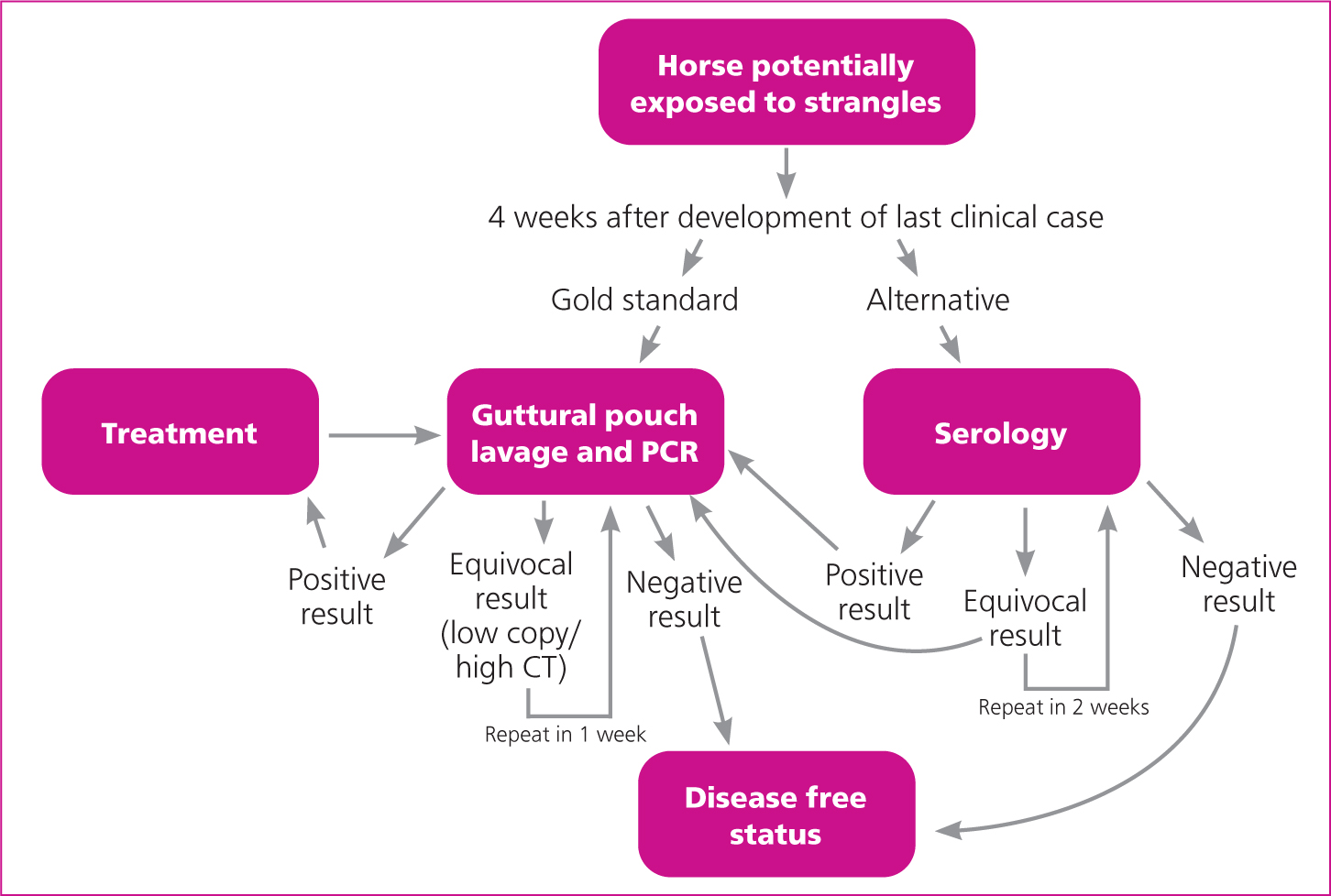
Culture-negative horses have been demonstrated to be a potential source of new outbreaks (Morris et al, 2020). The laboratory cut-off and definition of PCR ‘positive’ is important. Performing lavage too early also increases the risk that horses will be examined before lymph nodes have ruptured and be incorrectly diagnosed as free from infection. The authors would typically seek to investigate the presence of persistently infected horses 4 weeks after cessation of clinical signs of the last clinical case. Either i) all horses can be subject to guttural pouch lavage submitted for PCR or ii) as a minimum all horses that had clinical signs of strangles and all seropositive horses should be subject to guttural pouch lavage submitted for PCR. Horses that are equivocal on serology can either have serology repeated 14 days later or can be considered positive and guttural pouch lavage submitted for PCR (Figure 3). Although the authors are not aware of a recent direct comparison based on application of available quantitative PCR assays, they do not consider qPCR of three negative nasopharyngeal swabs to be a satisfactory alternative approach to qPCR of guttural pouch lavage samples for ruling-out the presence of persistent infection in the guttural pouches. Rather than considering the property as a whole, it is possible to manage horses as individuals or smaller groups and gradually declare freedom from infection, however this requires excellent biosecurity and comes with a risk of infection being transmitted back to horses previously declared free from infection.
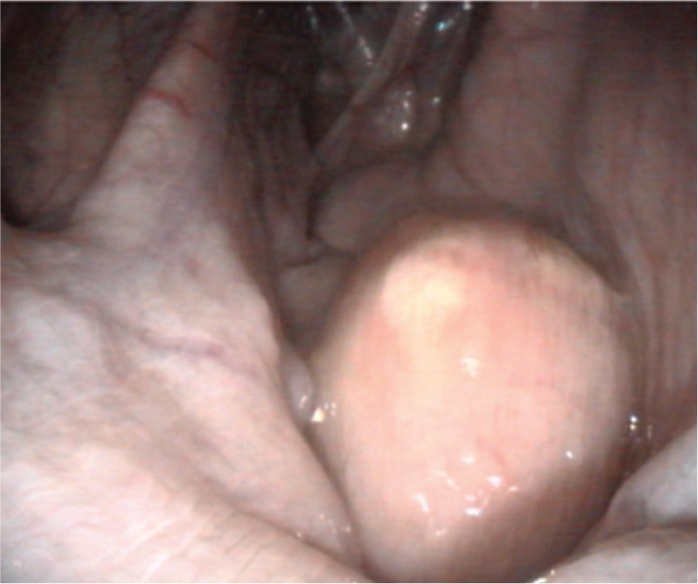
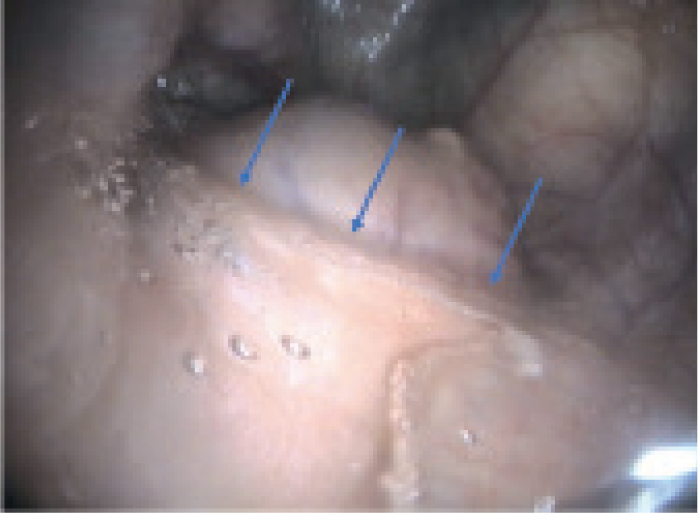
The approach to treatment may be influenced by endoscopic findings. For example, the presence of developing or draining abscesses (Figures 4 and 5) would indicate that it is too early to be attempting treatment. The approach to managing guttural pouch empyema will be determined by the nature of the material that is present within the pouch:

i)Purulent material
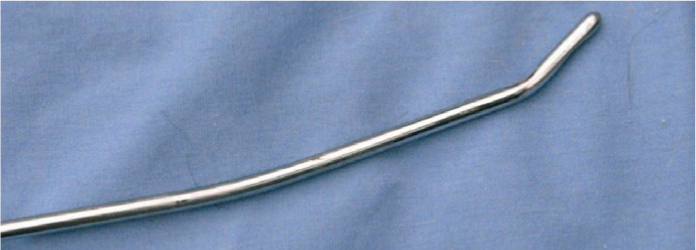
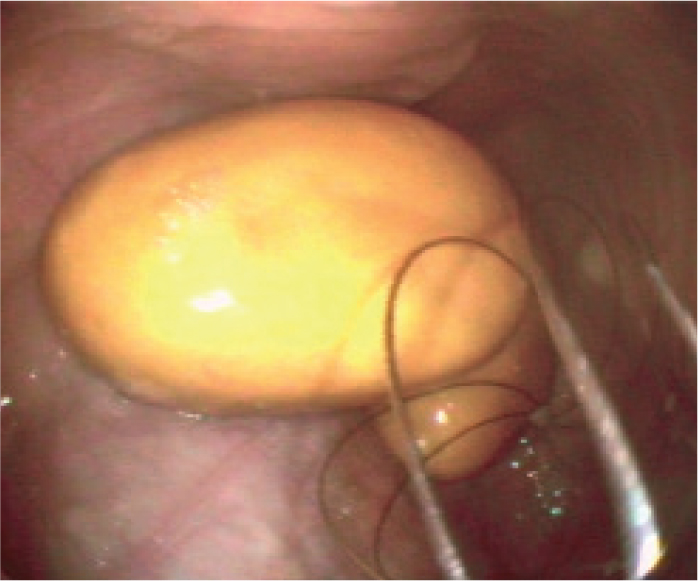
Purulent material that is liquid in nature can be lavaged from the pouch (Figure 6). Lavage channels of endoscopes are unlikely to be large enough to allow sufficient volumes to be infused. Lavage is best performed via insertion of a Nielson catheter and passage of a moderate to large volume of liquid into the pouch. Endoscopic guidance is not necessary. Foley catheters can be inserted and the cuff inflated to retain them in the pouch if repeated treatments are anticipated. It is necessary to place a wire (a dismantled metal coat-hanger or endotracheal stiffening wire is ideal for the purpose) through the centre of the catheter bent at around 30 degrees for the terminal 5 cm to facilitate passing the catheter through the guttural pouch ostium. Application of some lubricant around the wire facilitates its withdrawal when the catheter is in place. The cuff should not be left inflated during lavage as it will obstruct the outflow of the lavage fluid. If the catheter is being left in from day to day, then it should be inflated with water. If cuffs are inflated with air, they have a tendency to deflate allowing the catheter to fall out. If single treatments are anticipated, then plastic artificial insemination catheters can be used and bent toward the end if necessary, to facilitate their placement. Metal Nielson catheters (Figures 7a and 7b) are better for the purpose as they are angled, have rounded ends and pass easily into the pouch with or without endoscopic guidance.

The type of fluid that is used to lavage the guttural pouch is determined by clinician preference. Sterile saline would be the authors' preference, but water is a less expensive alternative and can always be made into an isotonic solution with the addition of electrolytes. Care should be taken with non-sterile water as one author has had a number of iatrogenic Pseudomonas infections in pouches that have been associated with significant morbidity. Some clinicians use dilute povidone iodine, but there is a concern that this can cause inflammation of the mucosa of the pouch and the cranial nerves that lie immediately beneath it.
Some pressure can facilitate lavage, but has to be applied very carefully to avoid rupturing the pouch with potentially fatal consequences. Pressure cuffs or pumps can be used with bags of saline, but garden pumps designed for applying herbicides or pesticides are equally effective. It is important that fluids are warmed to body temperature. Rupture of the guttural pouch has been reported so lavage needs to be performed with care (Fogle et al, 2007).
If small volumes of liquid material are present, then it should be possible to remove it in a single treatment. With larger volumes of purulent material, repeat treatments may be necessary. Treatment intervals are arbitrary. Where practical, lavage can be performed once or twice daily.
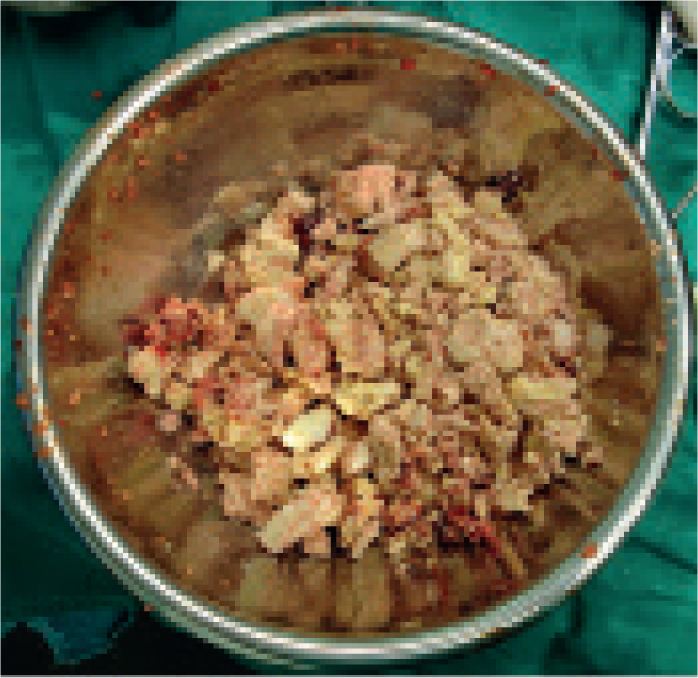
ii)Inspissated material
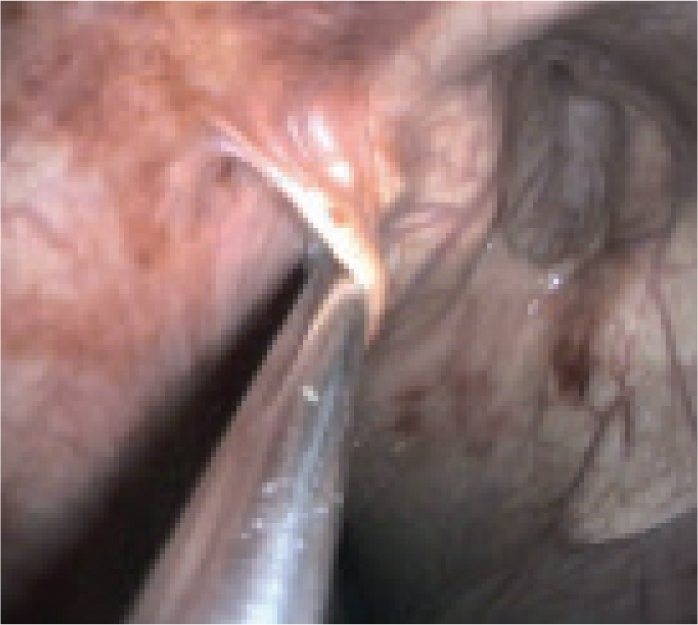
If purulent material has been present within the guttural pouch for extended periods it may become inspissated (Figure 8), forming discrete, separate chondroids over time. With chronicity these become hard and rounded and can be snared within a wire helical basket (Figure 9). With practice, and ideally an assistant, it becomes relatively straightforward to retrieve chondroids endoscopically. An effective helical basket is essential, as some are too soft and flexible. Acetylcysteine has been proposed as a means of breaking up inspissated material; however, most of the authors have found little benefit associated with its use (Verheyen et al, 2000). On rare occasions, chondroids are too large to fit through the guttural pouch ostium. These can sometimes be broken down with the baskets into smaller fragments. Rarely, surgical approaches have to be considered.
iii)Mixed empyema
When large quantities of material have been present for a few months there is often a mixture of soft ‘cheesy’ chondroids within more liquid purulent material. If the chondroids are too soft, small or numerous (and often all three) to be effectively clasped and removed, yet are also too large and firm to be lavaged from the pouch, then treatment can be challenging. One author (GH) has found that treatment of these cases has been revolutionised with the availability of reverse thermodynamic gel, which often results in development of more liquid purulent material that can be flushed out.
Treatment can be protracted and expensive, and, if finances are limited, it is worth considering isolating the horse for a few months in order to see if the material will become more inspissated and amenable to removal. Often, long-term isolation is impractical and/or undesirable for owners. An alternative is surgical opening of the pouch to remove infected material. Different approaches via Viborg's triangle have been reported under general anaesthesia and with standing sedation (Perkins et al, 2006). With the advent of surgical lasers, endoscopic approaches to create permanent fistulae between the guttural pouches and the pharynx have become possible (Hawkins et al, 2001) and are preferred by some clinicians to external surgical approaches. All approaches carry the risk of inadvertent damage of vital structures; but, on balance, the authors consider laser salpingotomy provides the best balance of benefit against risk. The majority of cases can be managed medically without the need for surgery.
Whether antimicrobials are helpful in the treatment of horses with guttural pouch empyema is unclear. While lymph nodes are abscessated or draining, antimicrobials are considered to be contraindicated. Thereafter, the use of antimicrobials is controversial but may hasten the resolution of infection in some cases. If there is a large amount of purulent material in the pouch there is little value in using systemic or instilled antimicrobials; the infected material needs to be physically removed. After this material has been removed it is commonplace for antimicrobials to be instilled into guttural pouches to eliminate any residual infection, hasten clinical resolution and reduce the probability that any subsequent samples will test positive. There is no evidence that the use of antimicrobials in this way will speed resolution, but it is logical that they might.
Eliminating the need for a further cycle of guttural pouch endoscopy and laboratory diagnostics provides a considerable cost saving in the management of the case. The authors have experience of cases that have had visual evidence of mucosal inflammation and have repeatedly tested culture and/or PCR positive following guttural pouch lavage and have then resolved following the instillation of penicillin, particularly when combined with reverse polymer that allows the antibiotic to remain in the pouch for 72 hours or more. It is speculated that biofilms might account for the persistence of infection in some cases.
To date, evidence of penicillin resistance has not been identified in strains of S. equi, but there is evidence of mutation of penicillin binding proteins (Harris et al, 2015; Morris et al, 2020) and this may lead to resistance in the future. It is important that antimicrobials are used sparingly and appropriately. Penicillin is the treatment of choice for local instillation into guttural pouches. Traditionally, a combination of sodium benzylpenicillin and gelatin has been used in the UK (Verheyen et al, 2000). This combination is expensive, and if the mixture is sufficiently liquid to pass easily into the pouch then it is also sufficiently liquid to exit. Procaine penicillin has been used as an inexpensive alternative although there is nothing to retain it within the pouch, and the instillation of procaine gives cause for concern when there are cranial nerves coursing superficially through the pouch. A small risk of antimicrobial-induced colitis has been reported in association with the use of penicillin in the guttural pouch when the solution is not retained in the pouch and is swallowed.
The use of sodium benzylpenicillin in a reverse thermodynamic gel that sets on contact with the mucosa of the guttural pouch (Figure 10) has been reported recently and is likely to maximise concentrations of penicillin in the pouch, while minimising the risk of adverse effects (Bowen, 2017). This combination is easier to use than penicillin dissolved in gelatin and has largely replaced this combination as it has been shown to be retained in the pouch for 72 hours or longer.
If systemic antimicrobials are considered, penicillin is the treatment of choice. Aminoglycosides and enrofloxacin are poor choices based on their likely spectrum of activity. Potentiated sulphonamides are ineffective in the presence of purulent material and, while they may appear effective based on the results of in vitro susceptibility tests, they may not be effective in vivo.
Doxycyline is an appropriate first-line alternative with good penetration and an appropriate spectrum of activity that may be used should an orally administered medication be required. Doxycycline hyclate is highly acidic, which may worsen pre-existing pharyngitis and it has poor palatability which is undesirable in horses that may already be inappetant. Doxycycline monohydrate is preferred as it has a neutral pH, is not irritant and is more palatable.
Preventing the spread of S. equi
It is important that quarantine periods are of sufficient duration to allow for the possibility of a protracted incubation period as well as uncertainty over when exposure occurred. Most premises will quarantine horses for 14 days, which is considered insufficient for strangles, and 28 days is considered optimal. A balance has to be struck between optimal disease control and owner compliance, and on this basis the authors advocate a 21-day quarantine period for all horses with consideration of 28 days quarantine in horses known to be at high risk of exposure to strangles.
Where finances permit, guttural pouch endoscopy can be used alongside quarantine to reduce the risk of introducing infection to a yard. If contact with S. equi in the preceding 28 days can be confidently ruled out, endoscopy and lavage/PCR of the guttural pouch can eliminate the possibility of carrier status and avoid the need for quarantine. Serology is a less robust alternative to sampling from the guttural pouches, as discussed above. Where guttural pouch endoscopy is being undertaken, veterinary surgeons should take particular care with hygiene, cleaning and disinfection around this procedure as it may act as a route of transmission of S. equi infection.
Outbreak management
This article has attempted to outline general principles for the management of strangles, but there is no prescriptive way to control a strangles outbreak. There are some general steps that can facilitate the implementation of any management plan, as the details of the plan for eliminating infection are less important than having the support of all relevant parties:
- Ensure that the messaging being provided to yard and horse owners is clear, consistent, and achievable.
- Ensure all involved (or at least as many people as possible) understand the reasons for the decisions taken. In the new era of online meetings and group messaging, it is possible to communicate with all of those affected simultaneously so everyone receives the same information.
- Where there is the potential for different approaches to be taken by different treating veterinary surgeons it is paramount that these are discussed, co-ordinated approaches are agreed and a unified front among veterinary surgeons and practices is maintained until the outbreak is resolved.
- It is worth considering appointing a lead veterinary surgeon for each yard outbreak to minimise the risk of conflicting messages being conveyed. This may be more challenging when there is more than one practice involved with treating horses on the yard but it should still be possible.
- Ensure practice reception and other support staff are aware of who is co-ordinating the management of the outbreak and ensure that they are either very well informed or simply relay messages to the central decision maker.
- Ensure the use of diagnostics is co-ordinated at a yard level and carefully integrated into the plan for eradication of infection. There is no need to perform confirmatory diagnostics on every case that shows clinical signs in an outbreak situation; these costs are better saved for post-outbreak clearance testing.
At the outset, hold a yard meeting for all those with horses on the premises and all those involved in treating them. Ensure that the veterinary staff have discussed this before an open meeting, have agreed the preferred approach and are not going to undermine confidence in the strategy by disagreeing in public. Prior to the meeting aim to designate one lead person and/or leadership structure that is ultimately responsible for the management of the horses on the yard, discuss the measures that need to be implemented and determine how they can be implemented in practice. At the meeting:
- Outline the disease situation on the yard.
- Ensure it is clear who on the yard is taking the lead for managing the outbreak and is the primary point of contact with the veterinary team.
- Ensure all members of the yard feel engaged in the decision-making process, feel they have had an opportunity to speak and feel that they have been listened to.
- Agree treatment strategies for acute cases.
- Agree biosecurity protocols.
- Agree the exit strategy that enables the yard to become disease-free.
- Answer questions openly and directly.
- Be realistic about the likely cost and timescale for eradicating infection to ensure expectations are managed appropriately and confidence is not lost when the reality does not reflect the initial advice.
The following measures should be considered when planning the response to an outbreak:
- Cessation of movements on and off the yard.
- Segregation of different groups of horses according to their disease status.
- Training of all those responsible for the care of the horses in basic biosecurity and nursing care; for example, hand washing, effective cleaning of equipment, using protective clothing and taking rectal temperatures.
- Establishment of traffic light-coloured groups reflecting disease status — red (confirmed infected and those with signs consistent with clinical strangles, being cognisant of comments above about cases not always being typical and not all requiring laboratory confirmation), amber (in contact with red group cases but no signs of disease), green (no contact with red group cases and no signs of disease).
- Taking and systematically recording rectal temperatures of all horses twice daily.
- Isolation of all horses with nasal discharge, cough, or temperature ≥38.5°C.
- Regularly empty, clean, dry and disinfect water troughs. Be explicit that addition of disinfectant to the water trough without emptying it is neither safe nor effective.
- Maximise the distance between groups and individual horses where possible. Even though S. equi is not airborne, increasing the distance between horses reduces the risk of disease transmission. Turning horses out and eliminating the need for feeding, mucking out, etc. within the stable reduces the risk of transmission on fomites and reduces the potential for S. equi to survive in the environment as it will be exposed to drier, lighter conditions. Horses that are turned out can be separated from one another with the use of double fence lines at least 3 metres apart to prevent nose to nose contact; electric tape is the cheapest and easiest option.
The following biosecurity measures should be considered for horses that are infected or potentially infected:
- Maximise distance from other groups and individual horses.
- Separate horses and ideally colour code with the traffic light system. Maintain separate equipment for each group and preferably each horse.
- Exclude all pets.
- Limit, and ideally dedicate, personnel dealing with the horses minimising the number of times they enter and exit.
- If use of limited and dedicated staff for infected horses is not possible, ensure personnel deal with ‘red’ horses last and shower and change before dealing with any other horses.
- Use suitable protective clothing and gloves that remain in the red/infected area other than being removed to be cleaned or destroyed.
- Dispose of bedding, uneaten feed and unused water in such a manner that it cannot come into contact with other horses or be picked up and transported by vermin.
- Provide facilities for washing and disinfecting hands and clothing, and instructions on how to thoroughly wash hands.
- Regularly disinfect contaminated areas and thoroughly deep clean and disinfect the whole area when it is no longer in use.
It is important that messaging is clear, concise, and consistent. Different options for action based on changing circumstances and levels or risk can be retained; however, it is essential that the reasons for differences are explained and expectations are managed from the outset. It is human nature to be inclined to conform to social norms, and this can be both a barrier and an opportunity. With careful, inclusive and values-based communication, owners can be engaged and supported to develop positive social norms around disease control and biosecurity. However, poor communication may result in owners and carers seeking the support of others who are aligned with their values and way of thinking, resulting in conforming to social norms that may not result in effective disease control.
It is particularly important that equine veterinary surgeons are observed by horse owners to carry out and adhere to effective biosecurity measures/practises, in relation to reducing transmission via clothing or equipment. In many cases, veterinary surgeons are considered to be role models by equine owners and carers, so they need to lead by example and demonstrate the disease prevention and control behaviours that need to be performed by all involved. Examples where this approach is seen and considered good practice is the livestock sector; an achievable goal for equine veterinary surgeons would be to change their own biosecurity behaviour alongside working with horse owners and carers, as has been achieved in much of the farming sector.
Equine veterinary surgeons also need to explain the biosecurity measures that are expected of owners and ensure the principles are understood; knowledge and skills are often assumed and while the principles of biosecurity may seem obvious to medical professionals, they are alien and complex to those without medical training.
Vaccination
A live attenuated submucosal vaccine (Equilis StrepE; MSD Animal Health) has been registered for use in the UK since 2004. Unfortunately, the uptake of vaccination in the UK is generally low compared to other European countries and this extends to vaccination for strangles. Horse owners see the risk of infection as being low and hence perceive a lack of value in vaccination, particularly when vaccines require administration every 3–6 months as they are with Equilis StrepE. Regrettably, the uptake of this vaccine has been hampered by a lack of familiarity with submucosal administration in the upper lip and a small risk of localised reactions, despite these reactions being mild and self-limiting. Isolated reports of abscessation when the vaccine is administered in association with other vaccinations or injections, reversion to virulence (Kemp-Symonds et al, 2007) and the possibility of reactions with inadvertent self-injection have also hampered efforts to encourage wider uptake of vaccination despite these risks being small. Current PCR and iELISA tests do not Differentiate Infected from Vaccinated Animals (DIVA) when the Equilis StrepE vaccine is used which can complicate the management of disease outbreaks.
A new multivalent component subunit vaccine for intramuscular vaccination has recently been developed and shown to reduce the severity of clinical disease (Robinson et al, 2018, 2020). It is currently pending approval as a registered product. This vaccine will have the advantage that it is derived from a more relevant strain, contains no live agent, is safe for administration via the intramuscular route and its use can be differentiated from natural infection (i.e. DIVA capability). However, like the current vaccine, it is anticipated that it will need to be administered every few months to provide optimal protection.
Consideration needs to be given to how the benefit of strangles vaccination can be explained to horse owners in a more effective manner. The cost of vaccination is small by comparison to the costs associated with the management of a clinical case let alone an outbreak. More positive and consistent messaging around vaccination is required.
Key Points
- Streptococcus equi has limited capacity for survival outside the horse but may persist for weeks in a dark, wet environment such as in a water trough.
- Following infection, there may be an incubation period of up to 4 weeks before the development of clinical signs.
- Severity of clinical signs will be determined by host immune status and challenge dose.
- If not investigated and treated, 75% of outbreaks will result in persistently infected horses that have the potential to cause other outbreaks.
- Pyrexia develops prior to nasal discharge and shedding of Streptococcus equi, providing an opportunity to isolate horses and prevent disease spread.
- 75% of infected horses will develop immunity to re-infection.
- Nasal discharge, pyrexia and mild lymphadenopathy are common clinical signs and are far more common than the classic signs of lymph node abscessation, dyspnoea and dysphagia.
- Streptococcus equi should be included in panels of laboratory tests for non-specific signs of respiratory disease.
- Ocular swelling, conjunctivitis, sinusitis, purpura haemorrhagica and myositis are uncommon clinical sequelae.
- Timing of swabbing acute cases is critical.
- Nasopharyngeal swabs are not recommended for the identification of carriers but are useful in detecting acute cases with nasal discharge.
- Nasopharyngeal swabs are preferred over nasal swabs and can be obtained from: equinesurveillance@gmail.com.
- Streptococcus zooepidemicus has the potential to cause guttural pouch empyema, but it is worth remembering the adage ‘if it looks like strangles then it probably is strangles’.
- Not all polymerase chain reaction (PCR) tests perform equally and care should be taken which test is being used. The inclusion of more than one gene target and a control target is optimal.
- Serological testing is most effective in the immediate aftermath of an outbreak.
- Serology will not detect all chronic carriers but remains a useful screening tool when guttural pouch endoscopy is not possible, as long as the limitations are understood.
- The dual target enzyme-linked immunosorbent assay (iELISA) has consistently been shown to be more reliable than the single target SeM iELISA.
- The strains circulating in Europe have changed over recent years, coinciding with decreased immune responses to SeM, and suggesting that their expression of this important protein may be reduced.
- The use of systemic antimicrobials should be avoided unless they are indicated in horses with dysphagia, dyspnoea or persistent pyrexia.
- Nursing care and anti-inflammatories are central to the management of acute cases.
- The recovery of more chronic cases can be expedited by removal of infected material from the guttural pouches by lavage, endoscopic removal or occasionally surgery.
- Instilling sodium benzylpenicillin in reverse thermodynamic gel into the guttural pouches can be considered once purulent material has been removed.
- Detection of carriers and effective quarantine measures for new horses are central to the control of strangles.
- Effective planning, communication and co-operation are essential in managing a strangles outbreak.
- Ensure all involved (or at least as many people as possible) understand the reasons for the decisions taken.
- Establish and implement practical and achievable biosecurity measures from the outset.
- A traffic-light system of grouping horses is practical and easy for all to understand.
- Do not assume knowledge of the principles of biosecurity and lead by example in observing them.
- Vaccination provides an exciting prospect for reducing the spread of strangles and severity of disease.
- More positive, effective and consistent messaging around vaccination is required.

